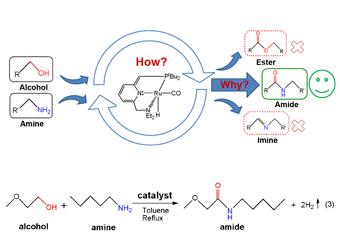
Using density functional theory (DFT) calculations, Wang’s group gave insight into the mechanism of the formation of amide from amines and primary alcohols, catalyzed by the pincer complex Ru(II)-PNN (PNN = 2-(di-tert-butyl-phosphinomethyl)-6-diethylaminomethyl) pyridine). The results lead us to propose a catalytic cycle that includes four stages: (Stage I) alcohol dehydrogenation to aldehyde, (Stage II) coupling of aldehyde with amine to form hemiaminal, (Stage III) hemiaminal dehydrogenation to amide, and (Stage IV) catalyst regeneration via H2 elimination of the trans Ru dihydride complex produced in the two dehydrogenation stages. Both of the dehydrogenation reactions proceed via the bifunctional double hydrogen transfer mechanism rather than the Beta-H elimination mechanism. The selectivity of amide over ester is determined by the coupling stage in which the aldehydeˆamine coupling to give hemiaminal is more favorable than aldehydeˆalcohol coupling to give hemiacetal. The competition between dehydrogenation and dehydration of hemiaminal governs the selectivity of amide over imine. Three alternative mechanisms without involving hemiaminal or hemiacetal have also been taken into considerations. One of them is less favorable than the pathway involving hemiaminal and the other two are unlikely although they have been shown to operate in other catalytic systems. The mechanistic difference is due to that alcohol dehydrogenation in the present system takes place via bifunctional double hydrogen transfer, whereas it prefers the β-H elimination mechanism in the other systems. The different dehydrogenation mechanisms are attributed to the different ways in which the catalytically active species are generated. In the current system, the catalyst is the catalytic active species itself, requiring no further activation, and its bifunctional active site makes the dehydrogenation follow the double hydrogen transfer mechanism. By contrast, the catalysts in the other systems need to be activated in situ to generate the active species that have vacant coordinate sites suitable for the -H elimination dehydrogenation.
The related results were published by
Organometallics (2011, 30, 5233-5247).
